High-temperature isotope fractionation
Overview
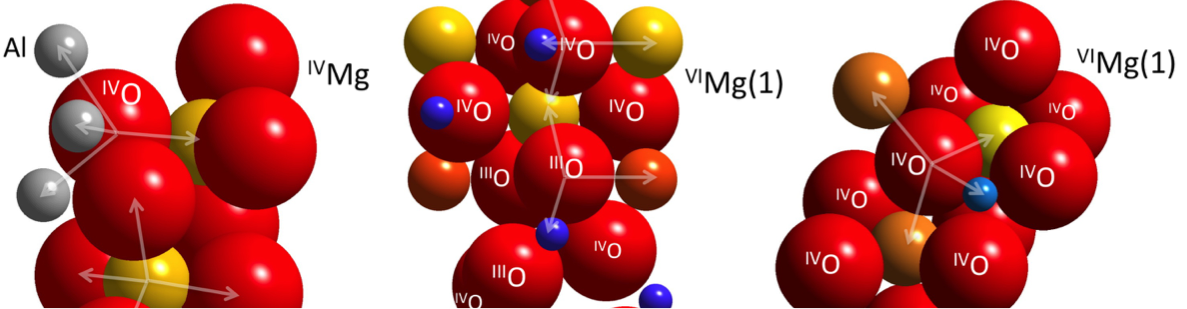
Our laboratory has engaged in measurements of stable isotope fractionation at high temperatures. High-temperature partitioning of the stable isotopes of rock-forming elements like Mg, Si, Fe, Ni and others are useful new tools in geochemistry and cosmochemistry. Understanding the fundamental driving forces for equilibrium inter-mineral fractionation comes from basic crystal chemistry and is invaluable for interpreting data from natural systems. Both charge and coordination number are key factors affecting bond length and bond stiffness and therefore the relative proclivity of a mineral phase for concentrating heavy or light isotopes. Quantitative interpretation of the plethora of new data relies on refinements of equilibrium fractionation factors through a feedback between crystal chemical reasoning, ab initio predictions, experiments, and analyses of well-characterized natural samples. This multifaceted approach is leading to a rapid rate of discovery using non-traditional stable isotopes in high temperature systems. For example, open-system mass transfer in the mantle is becoming increasingly evident from departures from equilibrium Mg and Fe isotope ratio partitioning between minerals, and differences in isotope ratios between bulk silicate Earth and meteorites are elucidating the conditions for planet formation quantitatively. These applications rely critically on accurate equilibrium fractionation factors.
Measurements of the silicon isotopic fractionation between metal and silicate are summarized in the figure below. These measurements were made by laser ablation MC-ICPMS (see Shahar et al. 2011 for details).
What we learn
For a summary of some applications, see the review by Young et al. (2015). One important application is the use of stable isotope fractionation to understand better the processes of planetary differentiation. The concept of using stable isotope ratios as proxies for the abundances of light elements in the core began in earnest with the paper by Georg et al. (2007) for silicon. The concept is straightforward. Isotope partitioning between Fe-Ni metal and silicate can cause a difference in isotopic composition between bulk silicate Earth and bulk Earth, the latter usually being assumed to be similar to chondrite meteorites. The general scheme in the case of Si is outlined in the figure shown below in which the relationship between the oxygen fugacity, temperature and pressure-dependent partitioning of Si between metal and silicate (i.e., core and bulk silicate Earth) is related to the offset in 30Si/28Si between bulk silicate Earth and bulk Earth (the latter assumed to be chondritic).